The Maye lab uses materials synthesis, nanochemistry, diffusion at nano interfaces, and biological programming to design and synthesize nanomaterials that have new function for energy, sensing, and biomimetic/bioinspired applications. Like any manufacturing process, it is only by controlling all aspects of a system, that unprecedented control and quality is possible. Over 22 Ph.D. students and postdocs and 35 undergraduate researchers have worked in our lab since 2008, and our work has been supported by the AFOSR, the NSF, and ACS.
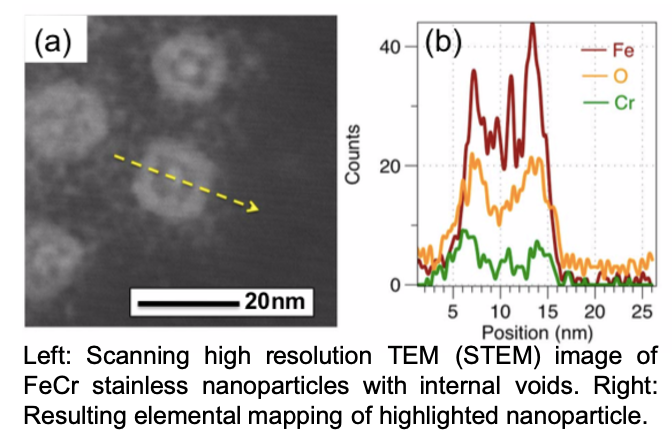
In our first project, we utilize materials chemistry adapted for nano-interfaces to chemically synthesize transition metal alloys with steel-like compositions and properties. In this work, we developed a novel “core-alloy” approach towards alloys, hypothesizing that (i) only the interface of a nanoparticle needs to the required alloy composition to adopt steel-like properties, and that (ii) alloying at the interface of nanoparticles may adopt different phase behavior and atom diffusion. To this end, our lab has been able to chemically synthesize “stainless steel” at the nanoscale, as well as a number of variants, and highly magnetic nanostructures. For example, the nanoparticles have shown resistance to oxidation (rusting) over prolonged periods of time in air, under heading, and when exposed to oxidizers. This work is ongoing, and a selection of published articles are show in articles 1, 2, 3, & 4. We also have patents and patents pending on the process, which have been licensed to outside companies.
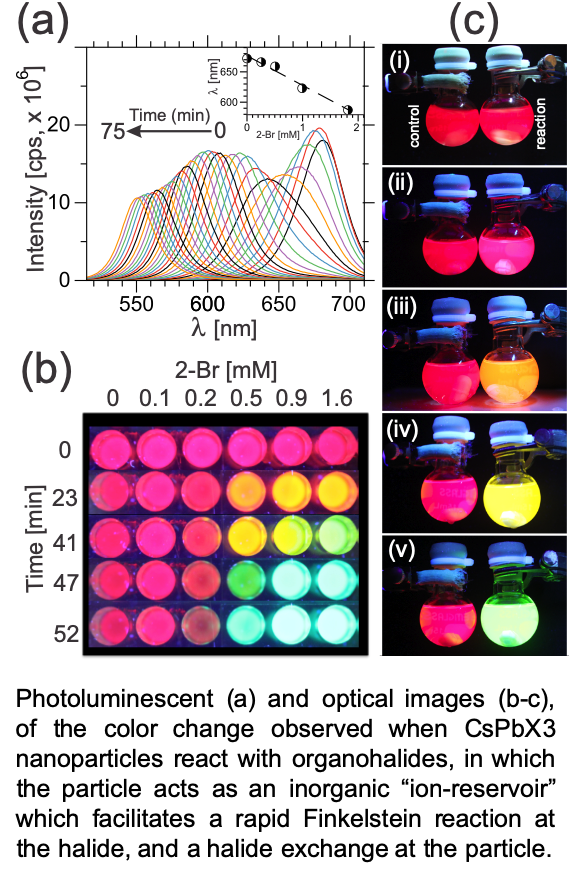
In a second project, we use nanomaterials as a novel “ion-reservoirs” for chemical reactions, as well as an in-situ color sensor/indicator for chemical reactions. Here, we employ all inorganic cesium lead halide (CsPbX3, X = F, Cl, Br, I) quantum dots, and utilize an atomic diffusion process called “halide-exchange” to drive chemical reactions or catalysis, as well as fluorescent color changes. For example, the figure above shows the remarkable color change that occurs when an organo-halide small molecule reacts with the CsPbX3. Analysis of the system shows that color change is a function of both the structure of the organo-halide as well as the surface structure of the CsPbX3. Of particular importance is that such color change can be observed at the single molecule level, meaning very low detection limits for halide containing small molecules, such as warfare agents. We have demonstrated this approach in articles 5 & 6 below, and also in a number of forthcoming articles. These processes are patent pending, and under consideration for licensing by outside companies.
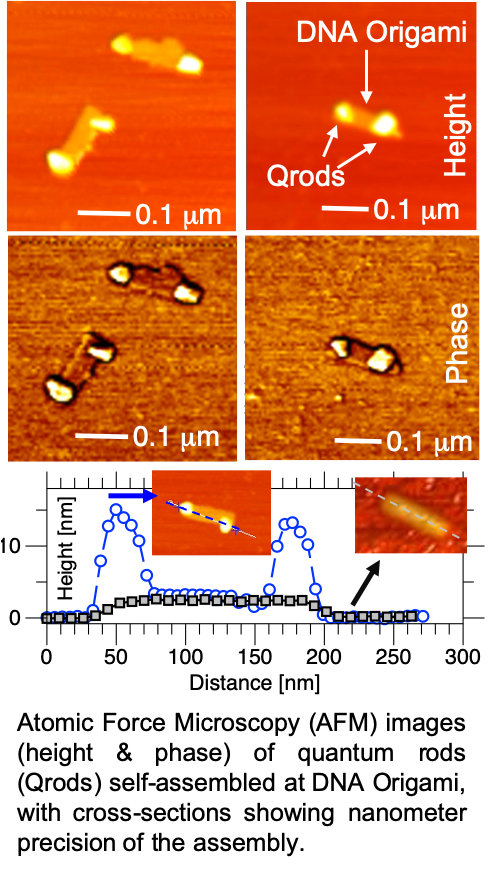
Our third project combines these nanomaterials with biomolecules, namely bioluminescent proteins or DNA, which then provides new opportunities for energy transfer or programming interactions. For instance, by binding luciferase enzymes to quantum rod interfaces, we have been able to show the highest bioluminescent energy transfer “BRET” efficiency, allowing the typically yellow/green light typical of bioluminescence, to be transferred to orange, red, or near infrared, depending on luciferase mutation, or quantum rod morphology or microstructure. This has been shown in articles 7,8,9 & 10 below, and also patented by our lab, which is available for licensing by outside companies. We continue to work in this area, focusing on how to increase the overall brightness of the bio-nano-conjugates, which have the potential as “turn-on” sensors, as well as solid-state bioinspired lighting.
In order to add even more function to these materials, we then utilize DNA-mediated programming for self-assembly or recognition. By adding single-strands of DNA, RNA, or PNA to the interface of the particles, they can recognize and bind with complementary sequences on surfaces, targets, or other particles. For example, by adding DNA to quantum dots of different colors (sizes) or rod morphology, we have been able to self-assemble “multi-color” cluster, which allow for the multiplexing of multiple targets. In a recent system, we have use DNA modified quantum rods to DNA Origami structures that we designed to organize the rods with different orientations & spacing. A few examples of this work is shown in articles 11,12,13,14, & 15 below. We have also used DNA-mediated systems for drug delivery of chemotherapy agents, creating novel new smart drugs. This work has been published in 16 & 17 below, and is patented and available for license.
We continue to work in this area, and are particularly interested in the self-assembly of rods, the incorporation of “smart” properties into the DNA design, as well as combining DNA recognition with the alloy and CsPbX3 particles described above.
Selected Publications
-
W. Wu, M.M. Maye* "Void Coalescence in Core/Alloy Nanoparticles with Stainless Interfaces" Small 2014, 10, 271-276.
-
L. Pathade, T.L. Doane, R.D. Slaton, M.M.Maye* "Understanding the Oxidation Behavior of Fe/Ni/Cr and Fe/Cr/Ni Core/Alloy Nanoparticles" J. Phys. Chem. C 2016, 120, 22035-22044.
-
R.D. Slaton, I. Bae, P.S. Lutz, L. Pathade, M.M. Maye* "The Transformation of a-Fe Nanoparticles into Multi-Domain FeNi-M3O4 (M = Fe, Ni) Heterostructures by Galvanic Exchange" J. Mater. Chem. C 2015, 3, 6367-6375.
-
P.S. Lutz, I.T. Bae, M.M. Maye* "Heterostructured Au/Pd-M (M = Au, Pd, Pt) Nanoparticles with Compartmentalized Composition, Morphology, and Electrocatalytic Activity" Nanoscale, 2015, 7, 15748-15756.
-
T.L. Doane*, K.L. Ryan, L. Pathade, K.J. Cruz, H. Zang, M. Cotlet, M.M.Maye* "Using Perovskite Nanoparticles as Halide Reservoirs in Catalysis and as Spectrochemcial Probes of Ions in Solution" ACS Nano 2016, 10, 5864-5872.
-
E.G. Ripka, C.R. Deschene, J.M. Franck, M.M. Maye* “Understanding the Surface Properties of Halide Exchanged Cesium Lead Halide Nanoparticles” Langmuir 2018, 34, 11139-11146.
-
R. Alam, L.M. Karam, T.L. Doane, K. Coopersmith, D.M. Fontaine, B.R. Branchini, M.M. Maye* "Probing Bioluminescence Resonance Energy Transfer In Quantum Rod – Luciferase Nanoconjugates" ACS Nano 2016, 10, 1969-1977.
-
R. Alam, D. Ablamsky, B. Branchini, M.M. Maye* “Designing Quantum Rods for Optimized BRET with Firefly Luciferase Enzymes” Nano Letters 2012, 12, 3251-3156.
-
R. Alam, J. Zylstra, D. Ablamsky, B. Branchini, M.M. Maye* “Novel Multistep BRET-FRET Energy Transfer using Nanoconjugates of Firefly Proteins, Quantum Dots, and Red Fluorescent Proteins” Nanoscale 2013,5, 5303-5306
-
R. Alam, L.M. Karam, T.L. Doane, J. Zylstra, D.M. Fontaine, B.R. Branchini, M.M. Maye* "Near infrared bioluminescence resonance energy transfer from firefly luciferase-quantum dot bionanoconjugates" Nanotechnology 2014, 25, 495606.
-
K.L. Hamner, C.M. Alexander, K. Coopersmith, D. Reishofer, C. Provenza, M.M.Maye* "Using Temperature Sensitive Smart Polymers to Regulate DNA-Mediated Nanoassembly and Encoded Nanocarrier Drug Release" ACS Nano 2013, 7, 7011-7020.
-
K. Coopersmith, H. Han, M.M. Maye* "Stepwise Assembly and Characterization of DNA Linked Two-Color Quantum Dot Clusters" Langmuir 2015, 31, 7463–7471
-
T.L. Doane, R. Alam, M.M. Maye* "Functionalization of Quantum Rods with Oligonucleotides for Programmable Assembly with DNA Origami" Nanoscale 2015, 7, 2883-2888.
-
M.M. Maye, K. Mudalige, D. Nykypanchuk, W. B. Sherman, O. Gang* “Switching binary states of nanoparticle superlattices and dimer clusters by DNA strands" Nature Nanotechnology, 2010, 5, 116-120.
-
M. M. Maye, M. Cuisinier, D. Nykypanchuk, O. Gang* “High Throughput Assembly of DNA-linked Nanoparticle Clusters” Nature Materials, 2009, 8, 388-391. (Cover Article)
Selected Patents:
- M.M. Maye, W.Wu “Method to Control Void Formation in Nanomaterials Using Core/Alloy Nanoparticles with Stainless Interfaces” US9604281B2 (2013)
- M.M. Maye “Compositions of Nanoparticles with Radial Gradients and Methods of Use Thereof” W02017087744A1 (2015)
- M.M. Maye, R. Alam “Bioluminescence Resonance Energy Transfer Between Bioluminescent Proteins and Semiconductive Nanomaterials” US9758808B1 (2012)
- M.M. Maye, T.Doane “System and Method for Visualizing Chemical Reactions in Real Time” US20180284086A1 (2015)
- M.M.Maye, J. Dabrowiak, C. Alexander “System and Method for Delivery of DNA-Binding Chemotherapy Drugs Using Nanoparticles” US8632789B2 (2010)